The Kenyan health authorities have issued an order to the public to immediately halt the consumption and circulation of a cancer drug after a concern about the suspicious appearance of the content of the drug on the market.
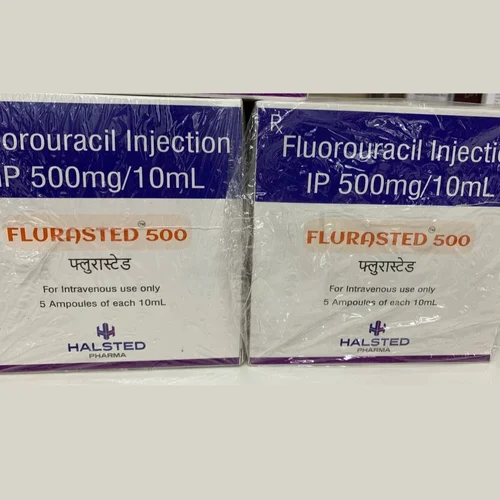
The Flurasted 500 (5-Fluorouracil) Injection Batch No. HHP24017, manufactured by Halsted Pharma Limited, an Indian company, is used for the management of cancer. It is commonly used in chemotherapy to treat cancers such as colon, breast, and stomach cancers.
However, numerous complaints reached the Ministry of Health offices in Kenya over the appearance of the content of the drug on the market, causing confusion and distortion among the public.
In response to these complaints, the Pharmacy and Poisons Board (PPB) released a statement on December 5, 2024, ordering the immediate halt in the use of the Flurasted 500 (5-Fluorouracil) Injection drug on patients.
‘’The Board orders the quarantine of Flurasted 500 (5-Fluorouracil) Injection, Batch No. HHP24017, manufactured by Halsted Pharma Private Limited, India,” the statement read.
The Pharmacy and Poisons Board CEO, Fred Siyo, acknowledged the concern and urged the overall public and health workers to abandon the administration of the drug as it works on the permanent means of withdrawing it from the shelves.
“In light of this, the Board advises all pharmaceutical outlets, healthcare facilities, healthcare professionals, and members of the public to immediately quarantine the product batch and stop the further distribution, sale, issuance, or use of the affected batch,” he stated.
He advised the public to report any suspected cases of substandard medicines or adverse drug reactions to the nearest healthcare facility or directly to the Pharmacy and Poisons Board through the various media health channels.
In the wake of a similar previous discovery, the Board a few weeks ago on November 22, 2024, gave another order to recall a drug acknowledging a mix-up in the labeling and packing process of two medicines that resulted in a disparity in the power of both drugs.